After learning about how the plant kingdom keeps salt out and how fish deal with high salt concentrations, I would like to discuss how it affects us humans. All throughout the history the availability of salt has been important to civilization. As a matter of fact the word salary come from the Latin word for ‘salt’, because the Roman Legions were sometimes paid in salt.

Salt is all around us, in our food, underground, on the earth’s surface and oceans around us. Its existence is due to the dried up residues of ancient seas. It has many types and methods of production; white salt comes from evaporating ‘solution-mined’ brine inside pressure vessels. The salt we use for our roads comes from ancient salt deposits in the mines. Many places in the world use the sun to produce salt from ocean water. To put this in perspective, ocean water has a salinity of 3.5% that is 35 grams of solids per liter.
Salt though is used in almost everything we eat and in making of plastic, paper, glass, polyester, rubber and fertilizers to household bleach, soaps, detergents and dyes. But we still want to keep it out of lives in some cases. For example salt is bad for your cars, also to our body in large content and most importantly the same water used to make salt cannot be used for drinking.
Nature uses membranes to remove salts and filter water. We have many such processes as well; filters (sieves) where the size of the sediments filtered is determined by the pore size. The other process is reverse osmosis, where water is pushed through a dense non-porous active layer film on a porous support (2). Water purification faces a major challenge of high energy costs incurred by current membrane systems to recover water from saline sources. These processes are costly as they require high osmotic pressure to push water through the membranes (2).
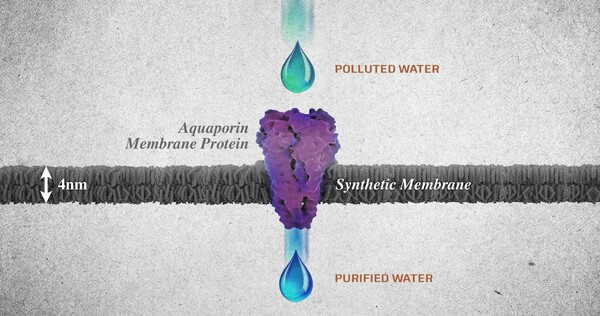
Nature uses aquaporins, also known as the water channel membranes, found abundantly in many of the mechanisms they are the functional unit of nature’s water purification systems. These membranes can be found in all the living organisms from mangrove plants to bacteria and human kidneys. Aquaporins work in an ingenious way in selective water transport. These molecules, basically attract the water molecules, due to the hydrophobic nature of the water channels, they shoot out the water molecule at the other end. In spite of water molecules being able to pass through the protein channel in a single file, this is highly effective and fast transportation of up to one billion every second.
One of the major advantages of such membranes is that they allow large volumes of water molecules to pass through a relatively small area at low pressures. Hence allowing for desalination of water at low pressure. Such membranes are responsible for filtering 90% of the salt in mangrove trees and providing up to 150 liters of filtered water in human kidneys. Apart from filtering salt aquaporin molecules have the ability to restrict the passage of contaminants including bacteria, viruses, minerals, proteins, DNA, dissolved gases, salts, detergents, and even protons without encumbering the passage of water (4).
Researchers at the National university of Singapore (NUS) have designed such a membrane which they claim is inspired from mangrove trees membranes. They have succeeded in placing these aquaporins proteins onto polymer filtration surfaces, which allow high volumes of water at low pressure and energy.
Assoc. Prof. Tong from NUS explains that “This biomimetic membrane is build to mimic the layers of cells on the roots of mangrove trees. This is done by embedding nano sized aquaporin vesicles onto a sable ultra filtration substrate membrane using an innovative yet simple and easy-to-implement surface imprinting technology”.
In contrast, due to the presence of two different layers, the biomimetic membrane presents a higher mechanical strength and stability. This makes it ideal for industrial applications where it could resolve large water related issues.
In conclusion, natural mechanisms mangrove trees and aquatic life provide inspiration for such biomimetic products. Further development of this could have serious implementations in biological and biomedical fields.
The many uses of Salt. (2017). Maldonsalt.co.uk. Retrieved 2 July 2017, from http://www.maldonsalt.co.uk/About-Salt-The-many-uses-of-Salt.html.
Biomimetic Membranes: Taking on Energy Usage in Water Purification. (2017). Waterworld.com. Retrieved 2 July 2017, from http://www.waterworld.com/articles/wwi/print/volume-27/issue-3/editorial-focus/desalination/biomimetic-membranes-taking.html
Highly efficient nature-inspired membrane could potentially lower cost of water purification by 30 per cent. (2017). Phys.org. Retrieved 2 July 2017, from https://phys.org/news/2015-12-highly-efficient-nature-inspired-membrane-potentially.html
Biomimetic Membranes: Nature Inspires Next Generation of Water Filtration Technology. (2017). Waterworld.com. Retrieved 2 July 2017, from http://www.waterworld.com/articles/iww/print/volume-14/issue-3/features/biomimetic-membranes.html
Repost from Keine Kommentare
 吉ICP备11002416号-2
吉ICP备11002416号-2

Add a comment: